Alkene Synthesis (Part 3)
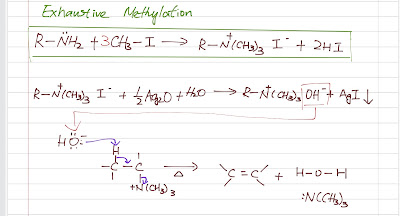
Hoffman elimination and Cope elimination are amine chemistry. Both reactions are concerted and both favor the Hofmann product (less-substituted alkene). Hofmann Elimination Leaving group The general form of an amine is R-NH2. The amide ion is a strong base hence a poor leaving group. So if we want an amine to undergo an elimination, we have to make a better leaving group first. We do this by exhaustive methylation (usually with methyl iodide) to convert the leaving group into a quaternary ammonium salt which can leave as a neutral amine. E2 Mechanism Hofmann elimination follow a E2 , concerted reaction mechanism which needs a strong base . The geometry is specific here (like a typical E2): anti-coplanar between the proton being abstracted and the leaving group. The quaternary ammonium salt is reacted with silver oxide to become a hydroxide salt to generate the strong base needed. Heat is applied and the Hofmann product is the major product. Hofmann Elimination Cop