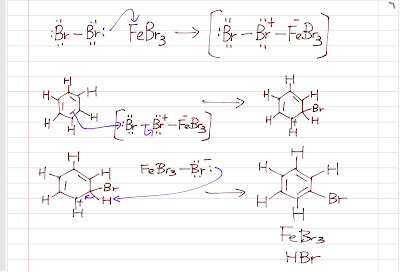
The first step of
EAS is highly endothermic as the aromaticity is lost. Therefore, we need a strong
electrophile to initiate the reaction. If we want to do
bromination, we cannot use Br2 directly because it is not a strong
electrophile (Br2 has no open
octect and it is nonpolar, no formal charges). We enhance its
electrophilicity by using a
Br2.FeBr3 (or
Br2.AlBr3)
intermediate (FeBr3 or AlBr3 is a
Lewis acid, electron acceptor, so it withdraws the electrons from Br, making it much more polar). The Fe-Br bond is more
polar so that the
Br is a stronger
electrophile.
The rest follows the general mechanism pattern. The electrophile attacks the benzene ring, forming a sigma complex. Then a proton is lost, giving off HBr and achieving aromaticity again. Chlorination is basically the same with the chlorine group instead of the bromine group.
 |
| EAS: Bromination |
The sigma complex is stabilized by resonance:
 |
| Sigma Complex |




Comments
Post a Comment