SN2: bimolecular nucleophilic substitution - Updated 2024!
This is probably the easiest among the four. What happens in this reaction is:
a nucleophile attacks
a leaving group leaves
which happens all at the same time.
We call this a concerted reaction. There is a transition state only and no intermediate is formed. Note that the carbon is electrophilic as it is next to a halogen which is electronegative.
A general form of the mechanism:
Here X represents a halogen; a halogen is always a decent leaving group.
Look at the red arrows as the electron flow: the nucleophile attacks from the back side of the molecule and the halogen leaves all in one step.
Look at the red arrows as the electron flow: the nucleophile attacks from the back side of the molecule and the halogen leaves all in one step.
We can see that the nucleophile can never be on the same side as the leaving group was. (a nucleophile cannot attack the same side as the leaving group leaves, they will crashhh together!)
As I mentioned, there is a transition state. And it is where the nucleophile almost forms a bond with the carbon while leaving group almost leaves the carbon. The transition state is an abstract state; You can't pause the reaction and extract the molecule while it is in the transition state.
The following is the transition state, not the product. Notice that the carbon almost form a 5 bonds structure.
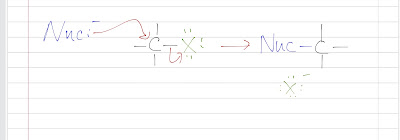




Comments
Post a Comment