SN1 SN2 Comparison
Knowing the reactions is not enough, we have to take a step further: to organize and compare them. There are several aspects that we can compare for the nucleophilic substitution reactions:
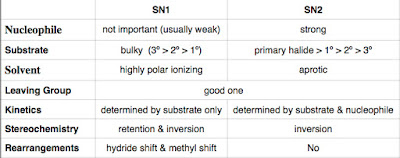
1. Nucleophile
SN1: Since a nucleophile is not involved in the rate-determining step, it does not have to be a strong nucelophile (in fact, a strong nucleophile will prefer a SN2 over SN1).
SN2: A strong nucleophile is required to make the concerted reaction feasible.
2. Substrate
SN1: As a carbocation intermediate determines the rate of reaction, a more substitued substrate is needed.
SN2: A crowded substrate will hindered the electrophilic carbon from the attack of the nucleophile. So a bulky substrate is a bad substrate for SN2.
3. Solvent
SN1: The ionization step is rate-determining, so a highly polar (ionizing) protic solvent stabilizes the ions formed.
SN2: It needs energy to strip off the solvent molecule if the nucelophile is solvated by the solvent. So aprotic solvent is better as no hydrongen bonding will be formed.
4. Leaving Group
Both reactions require a good leaving group.
5. Kinetics
SN1: the rate of reaction is determined by the concentration of the substrate ONLY as the ionization takes place in the substrate molecule.
SN2: the rate of reaction is determined by the concentration of both the substrate and the nucleophile because it is a concerted reaction.
6. Stereochemistry
SN1: There are two sides a nucleophile can attack the empty p orbitals of the carbocation intermediate. A racemic mixture may be formed. More often, there are more inversion product than the retention product.
SN2: The product formed is always from a back-side attack reaction. In other words, the nucleophile is at the opposite side of the leaving group.
7. Rearrangement
SN1: Hydride shift or methyl shift may occur to stabilize the carbocation intermediate before the nucleophile attacks.
SN2: As the reaction occurs in one step, there is no rearrangement can be formed.
Here is a table to summarize the comparison:
- Nucleophile
- Substrate
- Solvent
- Leaving Group
- Kinetics
- Stereochemistry
- Rearrangements
1. Nucleophile
SN1: Since a nucleophile is not involved in the rate-determining step, it does not have to be a strong nucelophile (in fact, a strong nucleophile will prefer a SN2 over SN1).
SN2: A strong nucleophile is required to make the concerted reaction feasible.
2. Substrate
SN1: As a carbocation intermediate determines the rate of reaction, a more substitued substrate is needed.
SN2: A crowded substrate will hindered the electrophilic carbon from the attack of the nucleophile. So a bulky substrate is a bad substrate for SN2.
3. Solvent
SN1: The ionization step is rate-determining, so a highly polar (ionizing) protic solvent stabilizes the ions formed.
SN2: It needs energy to strip off the solvent molecule if the nucelophile is solvated by the solvent. So aprotic solvent is better as no hydrongen bonding will be formed.
4. Leaving Group
Both reactions require a good leaving group.
5. Kinetics
SN1: the rate of reaction is determined by the concentration of the substrate ONLY as the ionization takes place in the substrate molecule.
SN2: the rate of reaction is determined by the concentration of both the substrate and the nucleophile because it is a concerted reaction.
6. Stereochemistry
SN1: There are two sides a nucleophile can attack the empty p orbitals of the carbocation intermediate. A racemic mixture may be formed. More often, there are more inversion product than the retention product.
SN2: The product formed is always from a back-side attack reaction. In other words, the nucleophile is at the opposite side of the leaving group.
7. Rearrangement
SN1: Hydride shift or methyl shift may occur to stabilize the carbocation intermediate before the nucleophile attacks.
SN2: As the reaction occurs in one step, there is no rearrangement can be formed.
Here is a table to summarize the comparison:


Comments
Post a Comment