More on SN1: stereochemistry and rearrangements
Stereochemistry
Unlike SN2 in which a nucleophile attacks only from the back, a nucleophile can attack the carbocation intermediate from either above or below because the carbocation has a planar, sp2 hybirdized orbitals structure. There are 2 ways the nucleophile can attack the empty p orbital.
When the nucleophile attacks in the side the leaving was, a retention product is formed. The nucleophile is where the leaving group was.
When the nucleophile attacks the other side, an inversion product is formed. The nucleophile is on the opposite side where the leaving group was.
Imagine in a shopping mall, there is only a door for both the people entering and leaving. This will create a big traffic. Therefore, there is usually more inversion product than the retention product (the leaving blocks the side as it leaves and the other side is always unhindered).
Rearrangement
When the carbocation intermediate is formed, a hydride ion or a methyl group will shift to form a more stable carbocation. For example, after a secondary carbocation is formed, a near hydride ion or methyl group will shift to provide a tertiary carbocation. A stable intermediate is preferred because it has a lower energy.
Carbocation stability: tertiary > secondary > primary
 | ||||||
| Hydride Shift |
 |
| Methyl Shift |
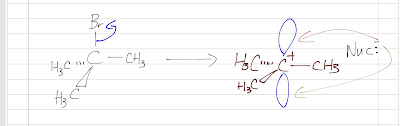



Comments
Post a Comment