Fischer Esterification of An Unknown Alcohol
As I love jumping from here and there, let's talk about Fischer Esterification!
While you may think that chemical reactions go in one way and never turn back. The truth is that a chemical reaction is always in an equilibrium!
Here is the Fischer Esterification, which is always a reaction between a carboxylic acid and an alcohol. Note that the source of the oxygen of the ester group and the reaction is always in an equilibrium.
Now, let's revise the concept of equilibrium and the math behind it like how to calculate the Keq.
We consider concentration when we look at an equilibrium. The initial concentration is Mi for both the acetic acid and the ethanol. When the equilibrium is established, a concentration of x of ester and water is formed. In a closed environment, the product can only be formed from the reactants. So the reactants lost a concentration of x to form the product. The final concentration of the reactants is Mi-x.
To calculate the Keq of the reaction, we divided the concentration of the products by the concentrations of the reactions. As the concentration of the ester formed is the same as the water formed, we can simply the product concentration using the square of the concentration of the ester only. Same put for the concentration of the reactants, we can replace the concentration by the concentration of the alcohol.
*The reason we are replacing the concentration using the ester and the alcohol is because water and carboxylic acid will dissolve together in the aqueous layer, while the other two will remain in the organic layer. By extraction, we can separate the organic layer out and do Chromatography to analyze their relative concentration.
*The reason that carboxylic acid is more dissolved in water rather than in the alcohol or the acid is that hydrogen bonding occurs between the carboxylic acid and the water.
The following is some useful notes related to this reaction. Btw, we add sulfuric acid to assure a sufficient proton source to catalyze the reaction or the equilibrium will not be established before a month or longer!
Here is the Fischer Esterification, which is always a reaction between a carboxylic acid and an alcohol. Note that the source of the oxygen of the ester group and the reaction is always in an equilibrium.
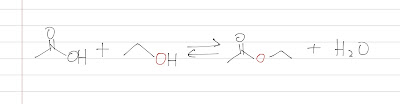 |
| Fisher Esterification |
We consider concentration when we look at an equilibrium. The initial concentration is Mi for both the acetic acid and the ethanol. When the equilibrium is established, a concentration of x of ester and water is formed. In a closed environment, the product can only be formed from the reactants. So the reactants lost a concentration of x to form the product. The final concentration of the reactants is Mi-x.
To calculate the Keq of the reaction, we divided the concentration of the products by the concentrations of the reactions. As the concentration of the ester formed is the same as the water formed, we can simply the product concentration using the square of the concentration of the ester only. Same put for the concentration of the reactants, we can replace the concentration by the concentration of the alcohol.
*The reason we are replacing the concentration using the ester and the alcohol is because water and carboxylic acid will dissolve together in the aqueous layer, while the other two will remain in the organic layer. By extraction, we can separate the organic layer out and do Chromatography to analyze their relative concentration.
*The reason that carboxylic acid is more dissolved in water rather than in the alcohol or the acid is that hydrogen bonding occurs between the carboxylic acid and the water.
The following is some useful notes related to this reaction. Btw, we add sulfuric acid to assure a sufficient proton source to catalyze the reaction or the equilibrium will not be established before a month or longer!
*we add NaHCO3 afterwards to neutralize the sulfuric acid left and assures it dissolves in the aqueous layer.
*the organic layer is above the aq. layer because it is less dense (less heavy) than the aqueous layer. Usually, when there is a dicloro-sth in the organic layer will it be below the aq. layer.
+ Further GC & NMR analysis is required





Comments
Post a Comment