Factors affecting rate of SN2
The are several factors that affect the reaction rate of SN2:
i. steric hindrance
ii. solvent effect
and 2 factors affecting the substrate
i. leaving group
ii. steric hindrance
So actually there are 4 factors affecting the reaction rate of SN2. Let's go over it one by one.
Nucleophile is a guy who loves nucleus as he has extra electrons around him. So generally, a nucleophile is negatively charged and the more negatively charge, the better nucleophile it is. We can then make a generalization that a conjugate base is a better nucleophile than its conjugate acid.
For example, an ethoxide ion is more nucleophilic than its conjugate acid, ethanol.
Common Pitfall: it is tempting to say that the ethoxide ion is more nucleophilic because it is more basic. WRONG! Basicity and Nucleophilicity is not the same. Basicity is the ability to get a proton, forming a bond to a proton while nuclophilicity is the rate a nucleophile can attack an electrophilic carbon atom.
Steric Hindrance:
Steric hindrance simply means crowdedness. A tert-butoxide ion is more hindered/bulky than an ethoxide ion. The methyl groups (blue circles) hinder the nucleophilic attack as they make the nucleophile crowded as shown. The ethoxide ion is less hindered because at the same carbon, it has hydrogen, which is smaller, instead of the methyl groups.
Common Pitfall: one may assume that as the tert-butoxide ion is a weaker nucleophile than the ethoxide ion, it must also be a weaker base. WRONG! Steric hindrance has very little effect on basicity because a proton is always that easy to grab; attacking a carbon which has formed 4 bonds already is obviously harder. Therefore, a bulky base doesn't mean a weaker base, but a bulky nucleophile means a weaker nucleophile.
Solvent Effect:
There are two types of solvent: protic solvent and aprotic solvent.
Protic solvent (typically alcohol)
A nucleophile is essentially an anion, so it forms hydrogen bonds with a protic solvent. This formation of bonds with solvent is called solvation. A solvated nucleophile needs energy to strip off the solvent before attacking the electrophilic carbon. This extra need of energy reduces the nucleophilicity. Also, a smaller nucleophile (fluoride ion) is more solvated than a bigger ion (iodide ion) simply because the hydrogen bonds formed is closer and stronger in a smaller anion than in a bigger one.
Recall the trend of polarization, which is that going down a column/ increasing the atomic number the polarizability increases because a big atom is easier to lose a small proton without even noticing. Same trend is true in the nucleophilicity in a protic solvent: With increasing atomic number, the nucleophilicity increases given a protic solvent environment because the solvation energy decreases with increasing atomic number.
Aprotic Solvent (e.g. alkane)
Aprotic solvent, on the opposite, enhances nucleophilicity because there is no hydrogen bond formation. So the anion is more reactive as it is less solvated. Such weak solvating ability has a
disadvantage: polar and ionic reagents are insoluble in an aprotic solvent.
Here comes the Polar Aprotic Solvents. Polar aprotic solvents have a strong dipole moment without acidic protons. The strong dipole moment enhances solubility and does not affect the nucleophilicity. Examples of polar aprotic solvents: acetonitrile, DMF, acetone.
A good leaving group enhances the rate of reaction. What makes a good leaving group? First, we have to understand what a leaving group does:
Steric hindrance:
Same thing regarding to crowdedness here again. If the substrate is corwded/ bulky, where can the nucleophile approach and attack? So a tertiary alkyl halides hardly undergoes a SN2. It is just too crowded that the nucleophile can't find a way to attack the electrophilic carbon atom from the back.
- Nucleophilicity (strength of nucleophile)
- Substrate (the guy being attacked by the nucleophile)
i. steric hindrance
ii. solvent effect
and 2 factors affecting the substrate
i. leaving group
ii. steric hindrance
So actually there are 4 factors affecting the reaction rate of SN2. Let's go over it one by one.
Nucleophilicity
Nucleophile is a guy who loves nucleus as he has extra electrons around him. So generally, a nucleophile is negatively charged and the more negatively charge, the better nucleophile it is. We can then make a generalization that a conjugate base is a better nucleophile than its conjugate acid.
For example, an ethoxide ion is more nucleophilic than its conjugate acid, ethanol.
Common Pitfall: it is tempting to say that the ethoxide ion is more nucleophilic because it is more basic. WRONG! Basicity and Nucleophilicity is not the same. Basicity is the ability to get a proton, forming a bond to a proton while nuclophilicity is the rate a nucleophile can attack an electrophilic carbon atom.
Steric Hindrance:
Steric hindrance simply means crowdedness. A tert-butoxide ion is more hindered/bulky than an ethoxide ion. The methyl groups (blue circles) hinder the nucleophilic attack as they make the nucleophile crowded as shown. The ethoxide ion is less hindered because at the same carbon, it has hydrogen, which is smaller, instead of the methyl groups.
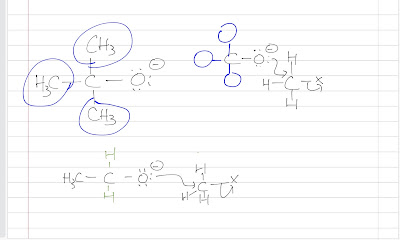 | |
| The one above is tert-butoxide ion and the one below is ethoxide ion |
Common Pitfall: one may assume that as the tert-butoxide ion is a weaker nucleophile than the ethoxide ion, it must also be a weaker base. WRONG! Steric hindrance has very little effect on basicity because a proton is always that easy to grab; attacking a carbon which has formed 4 bonds already is obviously harder. Therefore, a bulky base doesn't mean a weaker base, but a bulky nucleophile means a weaker nucleophile.
Solvent Effect:
There are two types of solvent: protic solvent and aprotic solvent.
- Protic solvent has acidic protons (usually exists in N-H or O-H groups) while aprotic solvent does not (aprotic solvent does not have N-H or O-H groups).
Protic solvent (typically alcohol)
A nucleophile is essentially an anion, so it forms hydrogen bonds with a protic solvent. This formation of bonds with solvent is called solvation. A solvated nucleophile needs energy to strip off the solvent before attacking the electrophilic carbon. This extra need of energy reduces the nucleophilicity. Also, a smaller nucleophile (fluoride ion) is more solvated than a bigger ion (iodide ion) simply because the hydrogen bonds formed is closer and stronger in a smaller anion than in a bigger one.
Recall the trend of polarization, which is that going down a column/ increasing the atomic number the polarizability increases because a big atom is easier to lose a small proton without even noticing. Same trend is true in the nucleophilicity in a protic solvent: With increasing atomic number, the nucleophilicity increases given a protic solvent environment because the solvation energy decreases with increasing atomic number.
 | |
| Don't look at this table as a periodic table; increasing atomic number means going down the periodic table |
Aprotic solvent, on the opposite, enhances nucleophilicity because there is no hydrogen bond formation. So the anion is more reactive as it is less solvated. Such weak solvating ability has a
disadvantage: polar and ionic reagents are insoluble in an aprotic solvent.
Here comes the Polar Aprotic Solvents. Polar aprotic solvents have a strong dipole moment without acidic protons. The strong dipole moment enhances solubility and does not affect the nucleophilicity. Examples of polar aprotic solvents: acetonitrile, DMF, acetone.
Substrate
Leaving Group:A good leaving group enhances the rate of reaction. What makes a good leaving group? First, we have to understand what a leaving group does:
- polarizes the bond it formed with the carbon and makes the carbon electrophilic
- leaves the electrophilic carbon with a pair of electrons that was once bonded with the carbon.
- a electron-withdrawing group (to polarize)
- a weak base (so it won't be reactive in it takes the electron away)
- polarizable (if not, the leaving group will be reactive during the transition state and the transition state will be an unstable. Therefore, a polarizable nucleophile also stabilizes the transition state)
Steric hindrance:
Same thing regarding to crowdedness here again. If the substrate is corwded/ bulky, where can the nucleophile approach and attack? So a tertiary alkyl halides hardly undergoes a SN2. It is just too crowded that the nucleophile can't find a way to attack the electrophilic carbon atom from the back.
Our team at Chemistery uses NordVPN every day and we highly recommend it.




Comments
Post a Comment