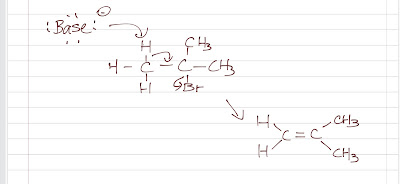
E2: Bimolecular Elimination
Here is what is going on in an elimination reaction:
Base & Substrate
Here, a strong base is required instead of a strong nucleophile because a proton is abstracted (not an electrophilic carbon atom being attacked). Note that a strong nucleophile is usually a strong base. If the substrate is a primary, SN2 may take place instead. Therefore, we want the substrate to be tertiary/ more substituted. This also has to do with the transition state and the alkene formed. In the transition state, a more substituted substrate is more stable, hence in a lower energy state. For the alkene formed, a more substituted alkene is the major product (Zaitsev's rule); when there is a choice of proton, the one that will form a more substitued alkene is favorably abstracted.
Stereochemistry
As the product is an alkene, the reaction must involve two carbons and the bond formation between them. There is a carbon that has the leaving group attached and the other has the proton being abstracted. Like SN2 (always back-side attack), E2 is a stereo-specific reaction too. The proton being abstracted has to be anti-coplanar (staggered conformation) to the leaving group.
- base abstracts proton
- pi bond forms
- leaving group leaves
 |
| E2 Mechanism |
Base & Substrate
Here, a strong base is required instead of a strong nucleophile because a proton is abstracted (not an electrophilic carbon atom being attacked). Note that a strong nucleophile is usually a strong base. If the substrate is a primary, SN2 may take place instead. Therefore, we want the substrate to be tertiary/ more substituted. This also has to do with the transition state and the alkene formed. In the transition state, a more substituted substrate is more stable, hence in a lower energy state. For the alkene formed, a more substituted alkene is the major product (Zaitsev's rule); when there is a choice of proton, the one that will form a more substitued alkene is favorably abstracted.
Stereochemistry
As the product is an alkene, the reaction must involve two carbons and the bond formation between them. There is a carbon that has the leaving group attached and the other has the proton being abstracted. Like SN2 (always back-side attack), E2 is a stereo-specific reaction too. The proton being abstracted has to be anti-coplanar (staggered conformation) to the leaving group.


Comments
Post a Comment