E1: Unimolecular Elimination
Similar to SN1, a carbocation intermediate is formed by an unimoleular ionization (rate-determining step). Then, a base (usually a weak base) abstracts a proton from the carbon which is next to the electrophilic carbon. A pi-bond is formed between these two carbons. Same as SN1, to stabilize the carbocation intermediate, a bulky/most substituted substrate is needed and rearrangements may occur. As the base attacks, the carbon next to the electrophilic carbon is rehybirdized to sp2 to form a pi-bond with the electrophilic carbon.
SN1 or E1?
Notice that both SN1 and E1 have the same unimolecular ionization first step and require a substituted substrate. So after the carbocation intermediate is formed, will the reaction go through the SN1 route or the E1? The answer is that we will have a mixture of E1 and SN1 product; the second step can either be a basic attack by the solvent (abstracts a proton to give an alkene) or an nucleophilic attack by the solvent on the carbocation.
Rearrangement
Methyl shift and hydride shift may occur to give a more substituted carbocation (same as in SN1). Note that in SN1, the solvent reacts like a nucleophile and in E1, the solvent reacts like a base.
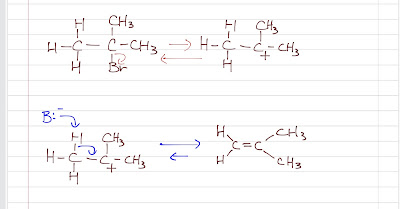 |
| E1 Mechanism |
Notice that both SN1 and E1 have the same unimolecular ionization first step and require a substituted substrate. So after the carbocation intermediate is formed, will the reaction go through the SN1 route or the E1? The answer is that we will have a mixture of E1 and SN1 product; the second step can either be a basic attack by the solvent (abstracts a proton to give an alkene) or an nucleophilic attack by the solvent on the carbocation.
Rearrangement
Methyl shift and hydride shift may occur to give a more substituted carbocation (same as in SN1). Note that in SN1, the solvent reacts like a nucleophile and in E1, the solvent reacts like a base.


Comments
Post a Comment