E1 E2 Comparison
Let's compare E1 and E2 in the following aspects:
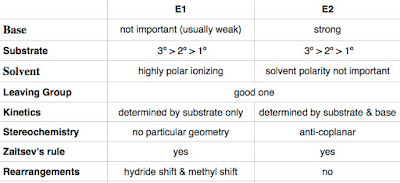
E1: As the base abstracts the proton only after the carbocation intermediate is formed, it does not affect the rate of reaction, hence not important.
E2: A strong base is indeed needed to promote the one-step reaction.
Substrate
E1: A more substituted substrate stabilizes the carbocation intermediate.
E2: A more substituted substrate forms a more substituted alkene.
Solvent
E1: A polarizing solvent enhances the rate of ionization as it pulls the cation and anion apart.
E2: The transition state is less sensitive to the solvent as the transition state has its negative charge shared over the whole molecule.
Leaving Group
Both reactions need a good leaving group.
Kinetics
E1: The rate is only determined by the substrate as it is the only molecule that ionizes.
E2: Both the substrate and the base affects the rate of the concerted reaction.
Stereochemistry
E1: It actually does not have a specific geometry, but it follows the Zaitsev's rule.
E2: As it is a concerted reaction, an anti-coplanar orientation between the base and the leaving group is required as it is the lowest energy state.
Rearrangements
E1: A methyl group or a hydride ion can shift to stabilize the carbocation intermediate before a base attacks.
E2: No rearrangements as there is no intermediate to be stabilized.
*Both E1 and E2 follow the Zaitsev's rule:
In elimination reactions, the most substituted alkene usually predominates.
Our team at Chemistery uses NordVPN every day and we highly recommend it.
- Base
- Substrate
- Solvent
- Leaving Group
- Kinetics
- Stereochemistry
- Rearrangements
E1: As the base abstracts the proton only after the carbocation intermediate is formed, it does not affect the rate of reaction, hence not important.
E2: A strong base is indeed needed to promote the one-step reaction.
Substrate
E1: A more substituted substrate stabilizes the carbocation intermediate.
E2: A more substituted substrate forms a more substituted alkene.
Solvent
E1: A polarizing solvent enhances the rate of ionization as it pulls the cation and anion apart.
E2: The transition state is less sensitive to the solvent as the transition state has its negative charge shared over the whole molecule.
Leaving Group
Both reactions need a good leaving group.
Kinetics
E1: The rate is only determined by the substrate as it is the only molecule that ionizes.
E2: Both the substrate and the base affects the rate of the concerted reaction.
Stereochemistry
E1: It actually does not have a specific geometry, but it follows the Zaitsev's rule.
E2: As it is a concerted reaction, an anti-coplanar orientation between the base and the leaving group is required as it is the lowest energy state.
Rearrangements
E1: A methyl group or a hydride ion can shift to stabilize the carbocation intermediate before a base attacks.
E2: No rearrangements as there is no intermediate to be stabilized.
*Both E1 and E2 follow the Zaitsev's rule:
In elimination reactions, the most substituted alkene usually predominates.
 |
| E1 E2 Comparison Chart |
Our team at Chemistery uses NordVPN every day and we highly recommend it.



Comments
Post a Comment