Basic Language of Organic Chemistry: E+ and Nuc:-
Every chemical reactions in organic chemistry are about nucleophile and electrophile. So a complete understanding of these two "philes" is fundamental for a further adventure in organic chemistry.
The suffix, "phile", means lover here. Nucleophile loves nucleus and electrophile loves electrons. That means, they are attracted to nucleus and electrons respectively. But why?
To make itself attracted to a nucleus, a nucleophile must be relatively negatively charged, so it always has a lone pair of electrons with it. That is why when we draw a generalized nucleophile, it has two extra electrons with it and it is negatively charged.
Same thing happens to an electrophile, it loves electrons, so it must be positively charged. And it is impossible to be negatively charged so it won't carry a lone pair of electrons around. In fact, stuff usually loses electron to become an electrophile. (electron deficient)
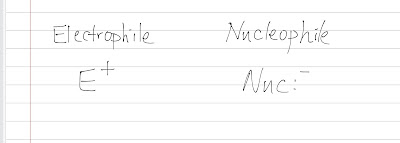


Comments
Post a Comment