Alkene Synthesis (Part 4)
Wittig Reaction
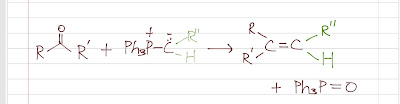
Wittig reaction turns a carbonyl (compound that has C=O) to an alkene by reacting a carbonyl (aldehyde or ketone) with a phosphorus ylide. It is a very useful reaction that turns a C=O to a desired C=C.
Making ylide
Let's talk about the phosphorus ylide. It has no overall charge, but it has a negatively charged carbanion that is bonded to a positively charged phosphorus. It is prepared by a two-step reaction sequence - an SN2 in which a triphenylphosphine attacking an unhindered alkyl halide (making a positively charged phosphorus), followed by a proton abstraction by a strong base (usually butyllithium).
We know that Phosphorus and Sulfur can form more than 4 bonds using the d orbitals. One may think that the ylide should have a double bond instead of having the carbon and phosphorus charged. The fact is that the pi bond between carbon and phosphorus is weak, so even though there is an existence of double bond, it is a minor resonance structure (yes, there are two resonance forms of phosphorus ylide).
Mechanism
Now, the ylide carbon atom is strongly nucleophilic. It can attack the carbonyl to form an intermediate (a betaine). A betaine has a positively charged phosphorus and a negatively charged oxygen. The negatively charged oxygen atom forms a strong bond with the positively charged phosphorus pretty fast because of the opposite charges, giving a ring structure called an oxaphosphetane ring (a four-membered ring). The ring then collapses, forming the alkene and triphenylphosphine oxide. The driving force of the reaction is the formation of triphenylphosphine oxide (a very stable compound) from triphenylphosphine.
Moreover, as an alkene is formed, the alkene product will be a mixture of cis and trans isomers if geometric isomerism is possible.
Retrosynthesis
It is common that we have to think backwards on how to make the alkene we want. The thought process is as simple as cutting the double bond into two pieces. We then determine which piece is from the ylide and which is from the carbonyl. Recall that we make the ylide from SN2 (reacting with an unhindered alkyl halide). Therefore, the piece that is less substituted comes from the ylide and the more substituted comes from the carbonyl. And the rest is just default operation (no thinking required if you practice enough).
Wittig reaction turns a carbonyl (compound that has C=O) to an alkene by reacting a carbonyl (aldehyde or ketone) with a phosphorus ylide. It is a very useful reaction that turns a C=O to a desired C=C.
Let's talk about the phosphorus ylide. It has no overall charge, but it has a negatively charged carbanion that is bonded to a positively charged phosphorus. It is prepared by a two-step reaction sequence - an SN2 in which a triphenylphosphine attacking an unhindered alkyl halide (making a positively charged phosphorus), followed by a proton abstraction by a strong base (usually butyllithium).
We know that Phosphorus and Sulfur can form more than 4 bonds using the d orbitals. One may think that the ylide should have a double bond instead of having the carbon and phosphorus charged. The fact is that the pi bond between carbon and phosphorus is weak, so even though there is an existence of double bond, it is a minor resonance structure (yes, there are two resonance forms of phosphorus ylide).
Now, the ylide carbon atom is strongly nucleophilic. It can attack the carbonyl to form an intermediate (a betaine). A betaine has a positively charged phosphorus and a negatively charged oxygen. The negatively charged oxygen atom forms a strong bond with the positively charged phosphorus pretty fast because of the opposite charges, giving a ring structure called an oxaphosphetane ring (a four-membered ring). The ring then collapses, forming the alkene and triphenylphosphine oxide. The driving force of the reaction is the formation of triphenylphosphine oxide (a very stable compound) from triphenylphosphine.
Moreover, as an alkene is formed, the alkene product will be a mixture of cis and trans isomers if geometric isomerism is possible.
Retrosynthesis
It is common that we have to think backwards on how to make the alkene we want. The thought process is as simple as cutting the double bond into two pieces. We then determine which piece is from the ylide and which is from the carbonyl. Recall that we make the ylide from SN2 (reacting with an unhindered alkyl halide). Therefore, the piece that is less substituted comes from the ylide and the more substituted comes from the carbonyl. And the rest is just default operation (no thinking required if you practice enough).






Comments
Post a Comment