Alkene Synthesis (Part 3)
Hoffman elimination and Cope elimination are amine chemistry. Both reactions are concerted and both favor the Hofmann product (less-substituted alkene).
Hofmann Elimination
Leaving group
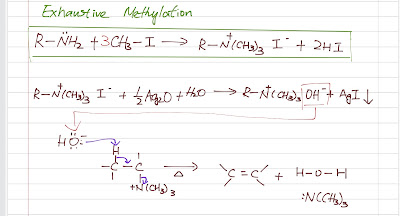
The general form of an amine is R-NH2. The amide ion is a strong base hence a poor leaving group. So if we want an amine to undergo an elimination, we have to make a better leaving group first. We do this by exhaustive methylation (usually with methyl iodide) to convert the leaving group into a quaternary ammonium salt which can leave as a neutral amine.
E2 Mechanism
Hofmann elimination follow a E2, concerted reaction mechanism which needs a strong base. The geometry is specific here (like a typical E2): anti-coplanar between the proton being abstracted and the leaving group. The quaternary ammonium salt is reacted with silver oxide to become a hydroxide salt to generate the strong base needed. Heat is applied and the Hofmann product is the major product.
Cope Elimination
Amine can be easily oxidized. In fact, amines are often converted to their salts for storage to prevent it from oxidizing. A tertiary amine can be oxidized to an amine oxide (using H2O2 or peroxyacid). There is a positive charge on nitrogen, so the amine oxide can undergo Cope Elimination. A strong base is not needed here because the amine oxide acts as the base and the transition state is cyclic. The cyclic transition state results in a syn stereochemistry of the product.
Hofmann Elimination
Leaving group
The general form of an amine is R-NH2. The amide ion is a strong base hence a poor leaving group. So if we want an amine to undergo an elimination, we have to make a better leaving group first. We do this by exhaustive methylation (usually with methyl iodide) to convert the leaving group into a quaternary ammonium salt which can leave as a neutral amine.
E2 Mechanism
Hofmann elimination follow a E2, concerted reaction mechanism which needs a strong base. The geometry is specific here (like a typical E2): anti-coplanar between the proton being abstracted and the leaving group. The quaternary ammonium salt is reacted with silver oxide to become a hydroxide salt to generate the strong base needed. Heat is applied and the Hofmann product is the major product.
 |
| Hofmann Elimination |
Amine can be easily oxidized. In fact, amines are often converted to their salts for storage to prevent it from oxidizing. A tertiary amine can be oxidized to an amine oxide (using H2O2 or peroxyacid). There is a positive charge on nitrogen, so the amine oxide can undergo Cope Elimination. A strong base is not needed here because the amine oxide acts as the base and the transition state is cyclic. The cyclic transition state results in a syn stereochemistry of the product.
 |
| Cope Elimination |



Comments
Post a Comment