Dehydration of alcohols
This is a reversible acid-catalyzed reaction. In fact, it is a common way to turn an
alkene into an alcohol. To increase the yield of the product, the
alkene produced is usually distilled off (an
alkene has a lower boiling point than its alcohol due to the lack of hydrogen bonding) to shift the equilibrium to the product side. Concentrated sulfuric acid is used as a catalyst to protonate the -OH group to a better leaving group, H2O. Then, a E1 mechanism is followed:
1. ionization (water leaves) to a crabocation
2. a weak base (water or HSO4-) abstracts the proton to form an alkene.
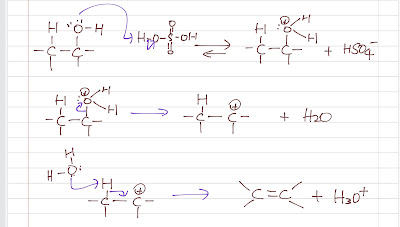 |
| Dehydration of alcohol |
Cracking (alkane)
An industrial (large scale and least expensive) way to make alkene is by the catalytic cracking of alkane (e.g. petroleum). A long chain of alkane is heated with catalyst (e.g. platinum) to form small alkenes (around 6 carbon atoms). This process of dehydrogenation is endothermic, but it has a positive entropy change.
 |
| Hydrogenation of alkane |
Addition Reactions of Alkynes
Alkyne is a hydrocarbon that has a carbon-carbon triple bond. During a reaction, the reagent adds across the triple bond to form an alkene (just like the addition reaction of alkene gives alkane). Here, I would like to talk about two types of hydrogenation that gives a
cis alkene and a
trans alkene respectively: Hydrogenation with Lindlar's catalyst and Metal-ammonia reducion.
1. Hydrogenation with Lindlar's catalyst
A Lindlar's catalyst is composed of barium sulfate coated with palladium and poisoned (partially deactivated) with quinoline. Two hydrogen atoms are added to the same face of the pi bond of the alkyne to make a
cis alkene. The usual platinum catalyst will turn the alkyne to alkane.
 |
| Hydrogenation of alkyne with Lindlar's catalyst |
2. Metal (sodium)-ammonia reduction
Sodium metal in ammonia solution reduces alkyne to a
trans alkene. When sodium dissolves in ammonia, it gives up electrons. This electron source essentially reduces the alkyne by adding it to the alkyne to form an radical anion. Ammonia acts as the proton source. It protonates two times to give the
trans alkene.
 |
| metal-ammonia reduction (step 1-2) |
 |
| metal-ammonia reduction (step 3-4) |







Comments
Post a Comment