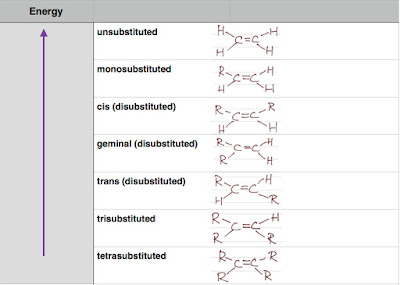
Stability of Alkene
From Zaitsev's rule, we know that a more substituted alkene is more stable.
For an alkyl disubstituted alkene, which of the cis or trans isomer is more stable? Since the substituted alkyl groups are further away from each other in the trans isomers, the trans isomers are usually more stable.
For a halogen disubstituted alkene, the trans isomer will have the dipole moment canceled out and the cis isomer with strong dipole moment as the electronegative halogen groups are on the same side.
Below is a table showing the energy of a series of substituted ethene. A higher energy alkene is less stable.
For an alkyl disubstituted alkene, which of the cis or trans isomer is more stable? Since the substituted alkyl groups are further away from each other in the trans isomers, the trans isomers are usually more stable.
For a halogen disubstituted alkene, the trans isomer will have the dipole moment canceled out and the cis isomer with strong dipole moment as the electronegative halogen groups are on the same side.
Below is a table showing the energy of a series of substituted ethene. A higher energy alkene is less stable.

Comments
Post a Comment